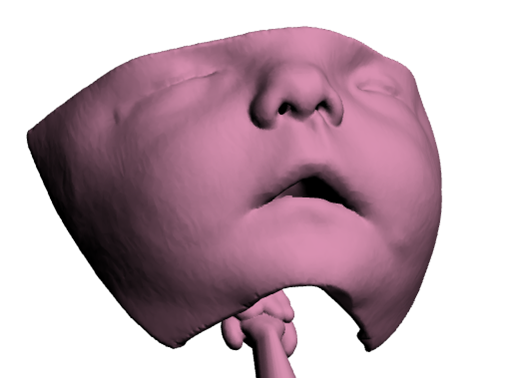
Customised non-invasive ventilation masks for children
Development of customised non-invasive ventilation interfaces for children for whom current commercial masks are unavailable or unsuitable to improve ventilation therapies and reduce complications.
Project Lead and Organisation
Professor Heather Elphick, Sheffield Children’s NHS Foundation Trust
When did project start?
October 2015
Clinical Requirement
Non-invasive ventilation (NIV) is the delivery of breathing support via a facemask. It is used to treat ineffective breathing and evidence shows that it improves quality of life and life expectancy. Ventilation is delivered through a mask covering the nose or nose and mouth. A good fit with a seal around the mask is needed to deliver the treatment effectively. Mass-produced masks are available for the adult market but in young children and infants it is often difficult to find a mask that fits adequately. A particularly disadvantaged group is children with facial deformities and facial asymmetry in whom NIV may not be possible due to unavailability of an adequate mask. The aims of NIV are to improve gas exchange and to improve respiratory symptoms. Without ventilation, these patients will suffer chronic hypoxia that can result in pulmonary hypertension, cor pulmonale (changes to the heart) and premature death. In some cases, clinicians have to resort to a tracheostomy in order to secure long term ventilation.
Care packages to support families that have home ventilation using a tracheostomy cost around £120,000 per year. If just one of these could be avoided, it would have significantly positive cost-implications for the NHS as well as huge improvements in the patient experience.
The Solution
We are exploring the use of innovative 3D assessment and manufacturing technologies to deliver novel mask-face interfaces to optimise mask fit to the needs of individual patients. The opinions and suggestions of patients and parents of children that use NIV are fundamental to the project design and are integral to the success of the project.
We have already demonstrated that the proposed method is more effective than a standard mass-produced mask in the laboratory setting. Potential business models will be evaluated to find the most commercially viable and clinically practical, and that offer the potential to scale up the use of this intervention on a national/international scale.
D4D are providing regulatory support, project management, and commercialisation guidance to this project.
Impact
The most important aspects for improvement (over standard treatment) as identified by children, parents and the clinicians were mask fit, sleep quality (by reducing disturbance caused by alarms and air leaks), comfort, and reduction of pressure sores. They felt such improvements would lead to a significant impact on both their medical condition (improved efficacy and compliance) and their quality of life.
Development of better NIV interfaces will also have a positive primary impact by improving NIV compliance and therefore therapeutic effectiveness. Further benefits are expected to occur through reductions in the incidence of unwanted effects such as skin breakdown. In addition, there are conservative predictions of potential cost savings to the NHS of £5 million per year. The technology could also be transferable to adult medicine, whereby the potential commercial impact and savings to the NHS will be substantial. The benefit to patients will be measurable in terms of improved treatment effectiveness, comfort and quality of life.
What is D4D’s role?
The paediatric TITCH network, managed by D4D, were approached to help develop this project. D4D were involved in producing the successful funding application for this project and have provided expertise throughout. This included the introduction of suitable collaborative partners, project management, developing exploitation and commercialisation strategies, and a pivotal role around the complex regulatory requirements for this project.
Partners
- NIHR Devices for Dignity HRC
- Sheffield Children’s NHS Foundation Trust
- Sheffield Teaching Hospitals NHS Foundation Trust
- Sheffield Hallam University
- The University of Sheffield
- Medipex Ltd
- Materialise Ltd
Funding
This research is funded by the National Institute for Health Research (Invention for Innovation, i4i; II-LB-0814-20004; Development of customised non-invasive ventilation interfaces for children for whom current commercial masks are unavailable or unsuitable to improve ventilation therapies and reduce complications.). The views expressed are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.