New therapy for post-stroke arm spasticity: Sheffield Adaptive Patterned Electrical Stimulation (SHAPES) – a co-designed system improvement followed by a powered multi-arm randomised control trial.
Project Lead and Organisation
Professor Wendy Tindale, Sheffield Teaching Hospitals NHS Foundation Trust & NIHR Devices for Dignity HRC
When did project start?
November 2020
Health & Care Requirement
85,000 people per year in the UK have a stroke, and numbers are predicted to double in the next 20 years. Strokes damage the brain, often making arm muscles hard to control. They become weak and then stay tight when they should relax, which is referred to as muscle spasticity. This over-tightness can become painful and disabling because muscles shorten and the arm cannot be moved freely.
Treatment options are limited. Available medicines have significant side-effects and can be expensive. Physiotherapy can help early on when in hospital but many patients are not offered it once they go home.
Project
The project builds on the work of Clinical Engineering at Sheffield Teaching Hospitals in the development of novel multi-channel stimulation devices for rehabilitation. Previous studies with small numbers of people who have had a stroke demonstrated promise for patterned forms of sensory stimulation as a therapy for post-stroke muscle stiffness (spasticity) affecting the elbow.
The aim is to investigate if a novel therapy (SHAPES) for relaxing tight arm muscles after stroke is better than two other treatments.
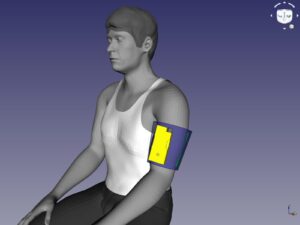
Design and Methods: The Sheffield team has developed a versatile new device (ShefStim-SBS) that sends tiny electrical pulses across 64 individual locations (SHAPES). It produces moving patterns of sensation – like being stroked. Other devices (TENS) work similarly but the sensation is only felt at 2 fixed locations – like tapping.
The first 12-months of the project will develop the ShefStim-SBS system further, with patient and public support, aiming to make it:
- Easier to wear and use at home
- Cheaper to manufacture
Years 2 and 3 will be focussed on completing the randomised control trial and in parallel continuing usability improvements informed by participants’ feedback.
We will have modified ShefStim-SBS to deliver either type of treatment (SHAPES or TENS) so that trial participants won’t know which type they are receiving to allow a blinded trial-design of the 2 interventions. People who have had a stroke and live with arm weakness will be recruited 2-16 weeks after stroke and receive one of three types of treatment for 6 weeks:
- Usual care + SHAPES
- Usual care + TENS
- Usual care
Impact
If the new therapy proves to be effective and offer value for money it could provide a new option for stroke rehabilitation for people who have had a stroke to use at home. It could reduce the long-term effects of arm spasticity, which limit independence and can be painful.
Partners
-
- Sheffield Teaching Hospitals NHS Foundation Trust (STH) (Study and trial sponsor), Clinical Engineering (Directorate of Medical Imaging & Medical Physics) & the Department of Clinical Neurology & Stroke PPI Group
- NIHR Devices for Dignity HRC
- University of Sheffield – School of Health & Related Research (ScHARR)
- Coventry University
- Barnsley NHS Foundation Trust
- Medipex Ltd
Project Sub-Contractors
-
- Centre for Process Innovation Ltd
- Daletech Electonics Ltd
- Talarmade Ltd
- Statistical Services Unit, University of Sheffield
Funding
This project is in receipt of £1,225,751 NIHR Invention for Innovation funding awarded from Call 19. More information is available from the NIHR’s website here.