Transdermal Fluid Removal – a novel community-based and patient self-administered treatment for fluid overload.
A large number of heart and kidney failure patients suffer from fluid overload that is resistant to diuretic tablets. Patients require hospitalisation to receive diuretic drips, and in extreme cases are put on dialysis. The purpose of this project is to develop a new medical device for the relief of fluid overload and oedema.
Project Lead and Organisation
Dr Idalia Dawidowska, Renephra Ltd
When did project start?
2009
Clinical Requirement
Chronic oedema affects 256,000 people in the UK and millions globally, and has a major impact on quality of life and mobility. Current treatment is based on compression bandages where limitations are; poor compliance, low efficacy, high costs and are ineffective in the treatment of central oedema.
There are also over a million patients with advanced heart failure worldwide, and 35,000 in the UK are admitted every year with decompensated heart failure with peripheral oedema [Ambrosy et al, 2014; CMFT Clinical Audit].
When treatment with oral diuretics (water pills) fails, as seen in 15-30% of advanced heart failure patients [Valente et al., 2014; Costanzo et al., 2005], high doses of intravenous diuretics (and in extreme cases dialysis) become necessary to remove excess fluid. This most often requires hospitalization, which is expensive and is not the preferred patient choice [British Heart Foundation study, 2015].
Renephra conducted a survey of 50 clinicians and nurses across various specialties closely involved in the care of patients with advanced heart failure with fluid retention. The survey revealed a clear dissatisfaction with the treatment options available. Existing treatments are invasive, expensive and give patients a poor quality of life.
The Solution
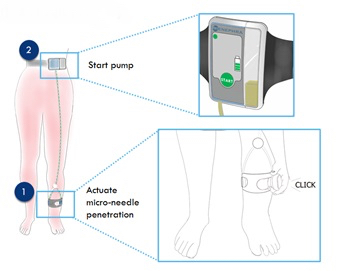
The team have developed a novel, cost effective, patented home/community-based method of removing fluid from the body that is gentler and potentially safer than intravenous diuretics, and that can be self-administered by patients. The proposed device removes excess fluid from the body by removing it from the skin’s interstitial fluid, the largest reservoir of fluid in volume-overloaded patients, sometimes harbouring up to 20 litres of excess fluid. The device uses microneedles to access fluid, and negative pressure to remove it. Patient tolerability is excellent with the intervention being completely painless, with patients finding it comfortable and unobtrusive.
The device is being developed for use in a home setting by patients and carers, so the need for hospitalisation or frequent clinic visits is expected to be reduced.
The team are currently working up the design and development of the prototype, ensuring it meets user requirements, to be ultimately ready for manufacturer and testing to be ready for CE mark. D4D will be supporting the project in developing the device in a user-centred way, with involvement of patients, carers, and clinicians. D4D are also responsible for working up an NHS adoption plan and internationalisation strategy to take the product to market.
Impact
Renephra’s approach has the potential to fulfil a huge unmet clinical and patient need by providing an opportunity for home based, gentle, “physiological” fluid removal to help manage symptoms, improve quality of life, prevent hospitalisations and improve outcomes. This approach offers a paradigm shift in the care pathway of advanced heart failure patients from predominantly hospital-based to mainly home/community-based care, leading to benefits such as better quality of life for patients, reduced kidney failure complications, and cost savings.
An early health economic evaluation of this new proposed method of fluid removal demonstrated a cost saving potential of up to £2,041 per decompensation episode in comparison to intravenous diuretics administered in hospital (at 80% success rate). There are around 35,000 admissions of people with decompensated heart failure with peripheral oedema each year in the UK.
Partners
- Renephra Ltd
- NIHR Devices for Dignity HRC
- Central Manchester NHS Foundation Trust / Manchester Health Ventures
- The University of Manchester
- Sheffield Teaching Hospitals NHS Foundation Trust
- Coventry University
- Stockcross Consulting Ltd
Patents
Granted:
- Europe (No.: 09708855.3)
- US (No.: 8585682)
- Australia (No.: 2009211201)
Pending:
- India (1831/MUMNP/2010)
- US (14/416,460)
The third patent (PCT/GB2015/050164) has recently entered national stage in:
- Europe (15701415.0)
- India (201617027603)
- Japan (still awaiting)
- US (15/113,249)